–°—А–Њ—З–љ–∞—П –њ—Г–±–ї–Є–Ї–∞—Ж–Є—П –љ–∞—Г—З–љ–Њ–є —Б—В–∞—В—М–Є
+7 995 770 98 40
+7 995 202 54 42
info@journalpro.ru
Anticorrosive effect of Delhi iron pillar.
–†—Г–±—А–Є–Ї–∞: –Ґ–µ—Е–љ–Є—З–µ—Б–Ї–Є–µ –љ–∞—Г–Ї–Є
–Ц—Г—А–љ–∞–ї: «–Х–≤—А–∞–Ј–Є–є—Б–Ї–Є–є –Э–∞—Г—З–љ—Л–є –Ц—Г—А–љ–∞–ї вДЦ4 2018» (–∞–њ—А–µ–ї—М, 2018)
–Ъ–Њ–ї–Є—З–µ—Б—В–≤–Њ –њ—А–Њ—Б–Љ–Њ—В—А–Њ–≤ —Б—В–∞—В—М–Є: 2109
–Я–Њ–Ї–∞–Ј–∞—В—М PDF –≤–µ—А—Б–Є—О Anticorrosive effect of Delhi iron pillar.
–†–µ–≤–∞—И–Є–љ –С–Њ—А–Є—Б –У–µ–љ–љ–∞–і—М–µ–≤–Є—З
–Я–µ—А–µ–≤–Њ–і —Б—В–∞—В—М–Є вАЮ–ѓ–≤–ї–µ–љ–Є–µ —Г—Б—В–Њ–є—З–Є–≤–Њ–є —Н–ї–µ–Ї—В—А–Њ—Е–Є–Љ–Є—З–µ—Б–Ї–Њ–є –Ј–∞—Й–Є—В—Л –Љ–µ—В–∞–ї–ї–Њ–≤ –Њ—В –Ї–Њ—А—А–Њ–Ј–Є–ЄвАЭ, –Њ–њ—Г–±–ї–Є–Ї–Њ–≤–∞–љ–љ–Њ–є –≤ вАЮ–Х–≤—А–∞–Ј–Є–є—Б–Ї–Њ–Љ –Э–∞—Г—З–љ–Њ–Љ –Ц—Г—А–љ–∞–ї–µвАЭ, –≤ вДЦ 9 –Ј–∞ 2015 –≥–Њ–і [6].
The phenomenon of stable electrochemical protection of iron from corrosion.
Key words: Anticorrosive effect; Delhi (Qutub вАФ Minar) iron pillar; anti вАФ corrosion protection.
There is Delhi iron pillar вАФ world heritage object.
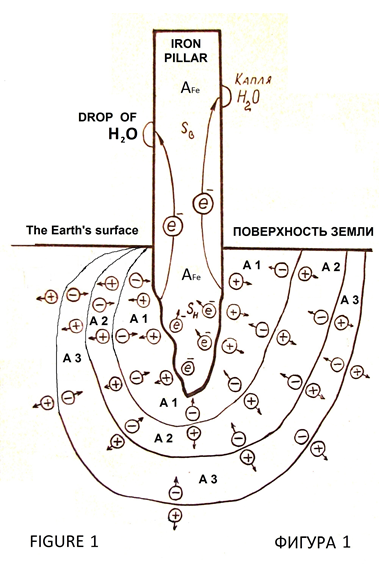
This iron pillar has no visible anti вАФ corrosion protection devices and stands more thousand years without corrosion [1]. In the twentieth century, one researcher sawed off a piece of iron from the pillar to determine the chemical composition of the metal. Bringing the sample to the lab, he found corrosion damage of metal [2, p. 122]. Consequently, anti вАФ corrosion resistance of Delhi iron pillar caused by under pillar of soil and geological features of the rock (Figure 1), [6]. Tools of modern industrial electronics allow reproduce the effect of corrosion resistance of the Delhi iron pillar.
The energy required to release an electron from the metal in a vacuum (in a state with a kinetic energy equal to zero), is called the emission constant, or the work of out [3, p. 107; 4, p. 129]. Emission constants there are in physics textbooks [4, pp. 160, 161]:
TABLE 1. Emission constants of iron Fe, silicium Si, aluminum Al, barium Ba, cesium Cs and cathode materials Ba-W, BaO-SrO, BaO-W, Ba-WO:
| Fe | Si | Al | –Т–∞ | Ba-W | BaO-SrO | –°s | Ba-O-W | Ba-WO |
| 4,36eV | 4,10eV | 3,74eV | 2,29 | 1,56eV | 1,37 | 1,36 | 1,34 | 1,10 |
Different metals, semiconductor materials have different values вАЛвАЛof the emission constants A. Combinations of metal films and oxide layers are used in electronics as cathodes for the emission of electron beams in a vacuum or gaseous medium. Film and oxide cathodes have a small amount of emission constant: 1.1 вАФ 2.5 electron вАФ volts [4, pp. 160, 161].
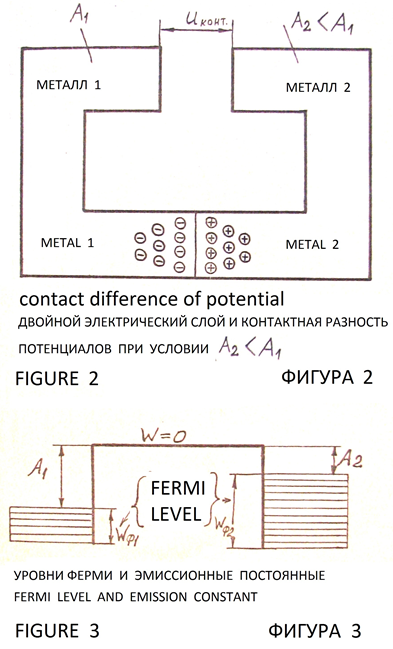
If two metals with different values of the emission constants to contact, then they make potential at the line of contact of metals by passing electrons from a metal with a lower emission constant into metal with a greater magnitude of the emission constant [3, pp. 111, 112], (Figures 2, 3). Theory and techniques of processes at the boundaries of materials with different values of the emission constants and contact potential difference can to explain, that the corrosion resistance of the Delhi iron pillar вАФ it is drift of charges from soil layers (geological rock or stony chalk, sand, clay) into the pillar, because there is difference of emission constants of electrons from the iron and materials under the pillar.
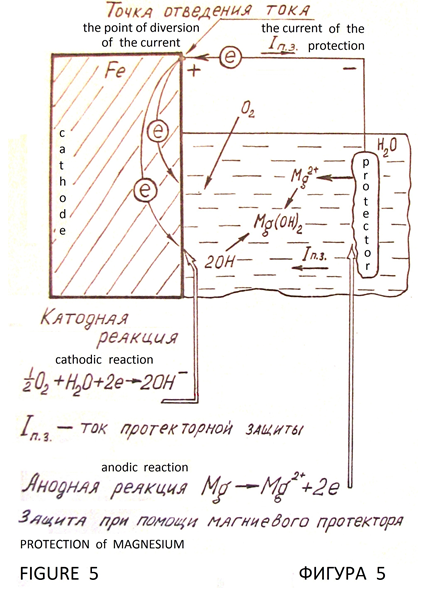
The drift of the electrons into the iron pillar creates capacity for sustained cathodic protection, supporting cathodic reaction during evaporation of individual droplets of atmospheric moisture. Delhi iron pillar can be called induced cathode without the traditional anode вАФ protector (Figure 1).
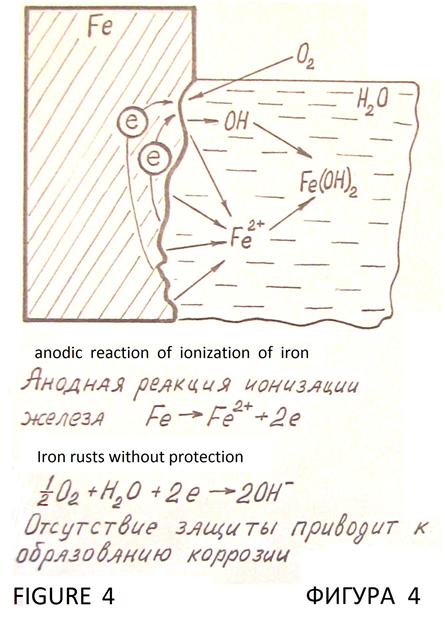
Without protection the iron rusts in a corrosive environment, such as in water (Figure 4).
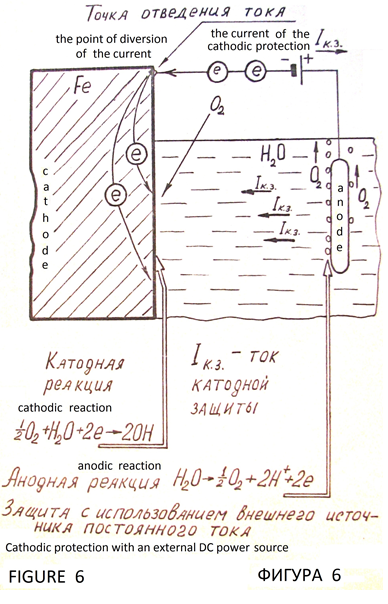
Cathodic protection of metals from corrosion is based on the cathodic polarization of the metal to the potential at which stops the process of ionization of the molecules of the metal. Galvanic cell is the source of polarizing current. Protected iron construction is the cathode. Electrode with positive potential вАФ is the protector (Figure 5). There is electrochemical protection with external DC power supply (Figure 6). Metal with virtually no corrosive, that is under complete cathodic protection. It is full protection. Potential, which is the full protection of the metal, is the protective potential, and the external current required to achieve it вАФ safety current. Obligatory condition of cathodic protection is the presence of conductive corrosion вАФ active medium (natural water, soil, and similar environments), and need maximum uniformity of the current distribution across the surface of the protected structure.
Criteria of cathodic protection systems are electrical values: safety protective potential and the current density. Stationary potential of steel in natural corrosion вАФ active media on average вАФ 0.55 volts relative mednosulfatnogo electrode. Polarization to the protective steel unit вАФ 0.85 Volt. This option was set in 1928, confirmed in the future long вАФ term supervision and now it is widely accepted criterion of cathodic protection of steel. The properly designed cathodic protection system provides 100% protection from corrosion [5, pp. 139 вАФ 143].
Systems of cathodic protection of metals from corrosion have a disadvantage: inversion of protection potential on long structures, enhanced corrosion at inversion of protection potential. A traditional, well вАФ known electrochemical system of protect metals from corrosion inappropriate to apply in cases of exposure to atmospheric moisture вАФ because low efficiency for it [5, p. 141]. Meanwhile, there is an example of corrosion resistance of Delhi iron pillar.
Under the Delhi iron pillar there are soil layers (of rock or chalk, or sand, or clay, or both, and the second and the third) as shown in Figure 1. The each layer of material has the emission constant A1, A2, A3. The value of the emission constant of iron –Р Fe =4,36 eV. The ratio of emission constants:
A Fe> A 1> A 2> A 3, or
A Fe> A 1> A 2 = A 3.
Such ratio of values emission constants of electrons provides the electrons to drift into a pillar from the underlying layers of вАЩвАЩfoundationвАЩвАЩ.
From the literature 3, pp. 110 вАФ 118 is known drift of electrons from a solid with lower value of the emission constant into the solid with the larger value of the emission constant. On the interface between solids arise contact difference of potential: Figures 2, 3.
Surface charges and contact difference of potential (Figures 2, 3) form a double layer of charges. Inside this layer there is an electric field, balancing the force of attraction of the electrons in the body with a greater magnitude of the emission constant and establishing equilibrium charge on contact plates of regular geometric shape (rectangular with small size). Contact potential, determined by the difference values вАЛвАЛof the emission constant of electrons in metals is from 0.1 to 3.0 V [3, p. 112].
In the Delhi iron pillar electrons accumulate at the base of the post and by mutual repulsion they drift to the top of the pillar, creating a surface excess electronic charge required for a protective electrochemical potential, which is the full electrochemical protection against atmospheric corrosion of iron. Structure of layers of foundation for the Delhi iron pillar is shown in Figure1. There may be other options. In the material under the base of the pillar the positive charges drift into the deep of earth due to the forces of repulsion of like charges to the border of materials A 1 вАФ A 2. Here there is an electric double layer (Figure 1) due to the difference value of the emission constant materials A 1 and A 2, the pillar turned negative charges into the ground вАФ positive, they are separated by the forces of mutual repulsion of the boundary materials A 2 вАФ A 3. The negative charge at the A1 вАФ A2 react with coming from the border AFe вАФ A1 positive charges mutually offsetting, so the border with A1 вАФ A2 resolve the charges and the reaction proceeds to charge separation schemes in Figures 2 and 3. A similar process of charge separation and the reaction of their compensation occurs at the interface of materials A2 вАФ A3.
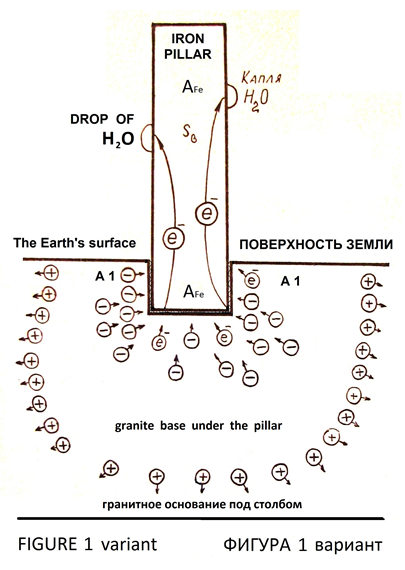
If the iron pillar was installed on a granite base, then granite base make the emission of electrons into the pillar as emitter, because in granite there is a concentration of electrons in certain parts of the volume on account of daily fluctuations in temperature and pressure (Figures 1, variant).
This concept of corrosion resistance of the Delhi iron pillar effect is open way to reproduce it by technical means.
Controlled combination of materials with different values вАЛвАЛof the emission constants of electrons makes the protective potential for electrochemical cathodic protection of iron plate by evaporation from the surface of a single drop of corrosion вАФ active liquid, isolated from the tread.
Modern technology applied cathode materials for metals could form an effective emission area for complete protection steel structures from atmospheric corrosion.
This article is translation into English of the article вАЮ–ѓ–≤–ї–µ–љ–Є–µ —Г—Б—В–Њ–є—З–Є–≤–Њ–є —Н–ї–µ–Ї—В—А–Њ—Е–Є–Љ–Є—З–µ—Б–Ї–Њ–є –Ј–∞—Й–Є—В—Л –Љ–µ—В–∞–ї–ї–Њ–≤ –Њ—В –Ї–Њ—А—А–Њ–Ј–Є–Є.вАЭ [L 6] by translator http://translate.google.ru.
Boris Revashin, 17, April, 2018.
Literature:
- https://annoyzview.wordpress.com/2011/12/27/rust-free-iron-pillar-of-qutub-minar/
- –У. –ѓ. –Т–Њ—А–Њ–љ–Ї–Њ–≤ вАЮ–≠–ї–µ–Ї—В—А–Є—З–µ—Б—В–≤–Њ –≤ –Љ–Є—А–µ —Е–Є–Љ–Є–ЄвАЭ –Ь., 1987 –≥.
- –§. –Ґ—Н–љ—Н—Б—Н—Б–Ї—Г, –†. –Ъ—А–∞–Љ–∞—А—О–Ї вАЮ–≠–ї–µ–Ї—В—А–Њ—Б—В–∞—В–Є–Ї–∞ –≤ —В–µ—Е–љ–Є–Ї–µвАЭ –Ь., –≠–љ–µ—А–≥–Є—П, 1980 –≥.
- –Ъ–Њ—И–Ї–Є–љ –Э. –Є –і—А.,"–°–њ—А–∞–≤–Њ—З–љ–Є–Ї –њ–Њ —Н–ї–µ–Љ–µ–љ—В–∞—А–љ–Њ–є —Д–Є–Ј–Є–Ї–µ" –Ь., "–Э–∞—Г–Ї–∞",1988–≥.
- "–Ґ–µ—Е–љ–Є–Ї–∞ –±–Њ—А—М–±—Л —Б –Ї–Њ—А—А–Њ–Ј–Є–µ–є " –Ѓ—Е–љ–µ–≤–Є—З –†. –Є –і—А., –Ы., вАЮ–•–Є–Љ–Є—ПвАЭ, 1980–≥.
- –†–µ¬≠–≤–∞¬≠—И–Є–љ –С. –У. вАЮ–ѓ–≤¬≠–ї–µ¬≠–љ–Є–µ —Г—Б—В–Њ–є¬≠—З–Є¬≠–≤–Њ–є —Н–ї–µ–Ї¬≠—В—А–Њ¬≠—Е–Є¬≠–Љ–Є¬≠—З–µ¬≠—Б–Ї–Њ–є –Ј–∞¬≠—Й–Є¬≠—В—Л –Љ–µ¬≠—В–∞–ї¬≠–ї–Њ–≤ –Њ—В –Ї–Њ—А¬≠—А–Њ¬≠–Ј–Є–ЄвАЭ, –Х–≤—А–∞–Ј–Є–є¬≠—Б–Ї–Є–є –љ–∞¬≠—Г—З¬≠–љ—Л–є –ґ—Г—А¬≠–љ–∞–ї вДЦ 9, —Б–µ–љ¬≠—В—П–±—А—М 2015 –≥., http://journalpro.ru/articles/yavlenie-ustoychivoy-elektrokhimicheskoy-zashchity-metallov-ot-korrozii/